Device Closure (ASD, PDA)
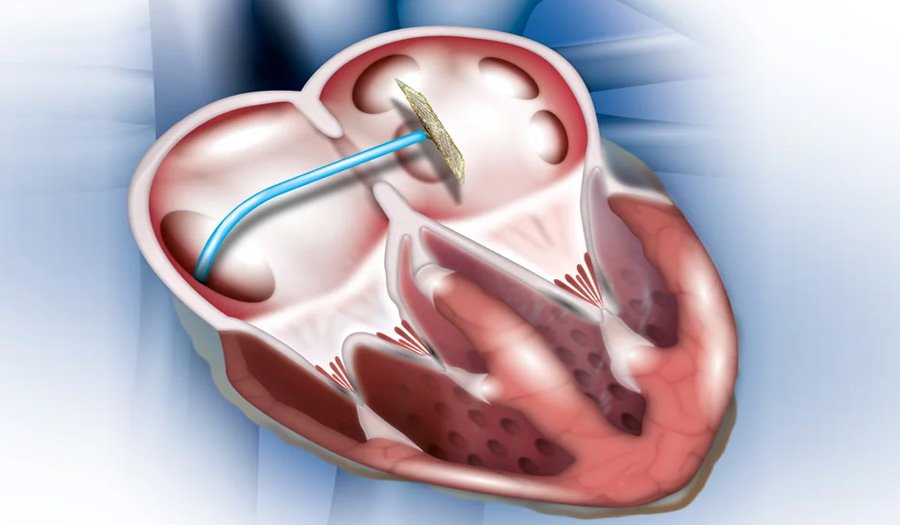
Doctors use device closure, a minimally invasive procedure, to correct congenital heart defects like Atrial Septal Defects (ASD) and Patent Ductus Arteriosus (PDA). These conditions disrupt normal blood flow due to abnormal openings in the heart, and device closure effectively restores function without requiring open-heart surgery.
What Is Device Closure?
In device closure, doctors place a specially designed device through a catheter to seal the abnormal opening in the heart. This procedure restores normal blood circulation and prevents complications such as heart failure, arrhythmias, or pulmonary hypertension.
Types of Defects Treated
- Atrial Septal Defect (ASD):
- A hole in the wall (septum) between the heart’s two upper chambers (atria).
- This defect allows oxygen-rich blood to mix with oxygen-poor blood, straining the heart and lungs.
- Patent Ductus Arteriosus (PDA):
- A persistent opening between the aorta and pulmonary artery that remains after birth.
- This defect leads to extra blood flow to the lungs, increasing the heart’s workload.
How Does the Procedure Work?
- Preparation: Doctors sedate the patient and access a vein in the groin area.
- Catheter Insertion: They guide a thin, flexible tube (catheter) to the heart using X-ray imaging and echocardiography.
- Device Placement: Doctors deliver the closure device, typically a self-expanding mesh or umbrella-like structure, to the defect site through the catheter.
- Sealing the Defect: The device expands and seals the hole, allowing the heart to function normally.
- Verification: Doctors confirm proper device placement using imaging before removing the catheter.
Who Needs Device Closure?
Doctors recommend device closure for patients who have:
- Large ASDs or PDAs causing symptoms like shortness of breath, fatigue, or heart murmurs.
- Recurrent respiratory infections or failure to thrive in children.
- Signs of heart enlargement or pulmonary hypertension due to untreated defects.
- No significant improvement with medication.
Advantages of Device Closure
- Minimally Invasive: It eliminates the need for open-heart surgery, allowing smaller incisions and faster recovery.
- Shorter Hospital Stay: Most patients leave the hospital within a day or two.
- Reduced Risks: It poses a lower risk of infection or complications compared to surgical methods.
- Quick Recovery: Patients resume normal activities sooner.
- High Success Rates: It effectively seals heart defects with long-term benefits.
Risks and Considerations
Although generally safe, device closure may involve:
- Bleeding or infection at the catheter insertion site.
- Rare cases of device dislodgement or improper placement.
- Allergic reactions to contrast dye or medications used during the procedure.
- Arrhythmias (irregular heartbeats), which usually resolve on their own.
Innovations in Device Closure
- Advanced Imaging Techniques: Ensure precision during device placement.
- Customized Devices: Designed to fit defects of varying sizes and shapes.
- Next-Generation Materials: Biocompatible devices reduce risks of rejection or erosion.
